
SMMA helped Cytiva find the perfect existing facility.
The company needed a location that would meet their current operational needs and provide flexibility for future growth. They selected a multi-story building in Marlborough with solid amenities and infrastructure capable of housing a range of cGMP, lab, and office environments.

The team explored multiple lab and office configurations, as well as conceptual designs that enhanced the building’s large, double-height space into an open forum for informal gatherings and meetings.
How we workshopped the layout
|SMMA held a workshop with 30 stakeholders to develop an office plan to meet Cytiva’s day-to-day needs. The team worked closely with Cytiva employees to understand departmental adjacencies, equipment and process requirements, and the goals of each business unit.

By the end of the workshop, the design team had produced a diagrammatic map. This map illustrated the ideal relationships among the multiple business units that were being consolidated into the new location.

Cytiva’s new labs are ground zero for scientific advancements.
Wet and dry labs support the company’s bioprocessing, chromatography, and cell therapy research. Specialized training and support labs foster collaboration and knowledge sharing.

Designed with adaptable infrastructure, the labs can accommodate future scientific needs and ensure Cytiva stays competitive in a rapidly evolving field.

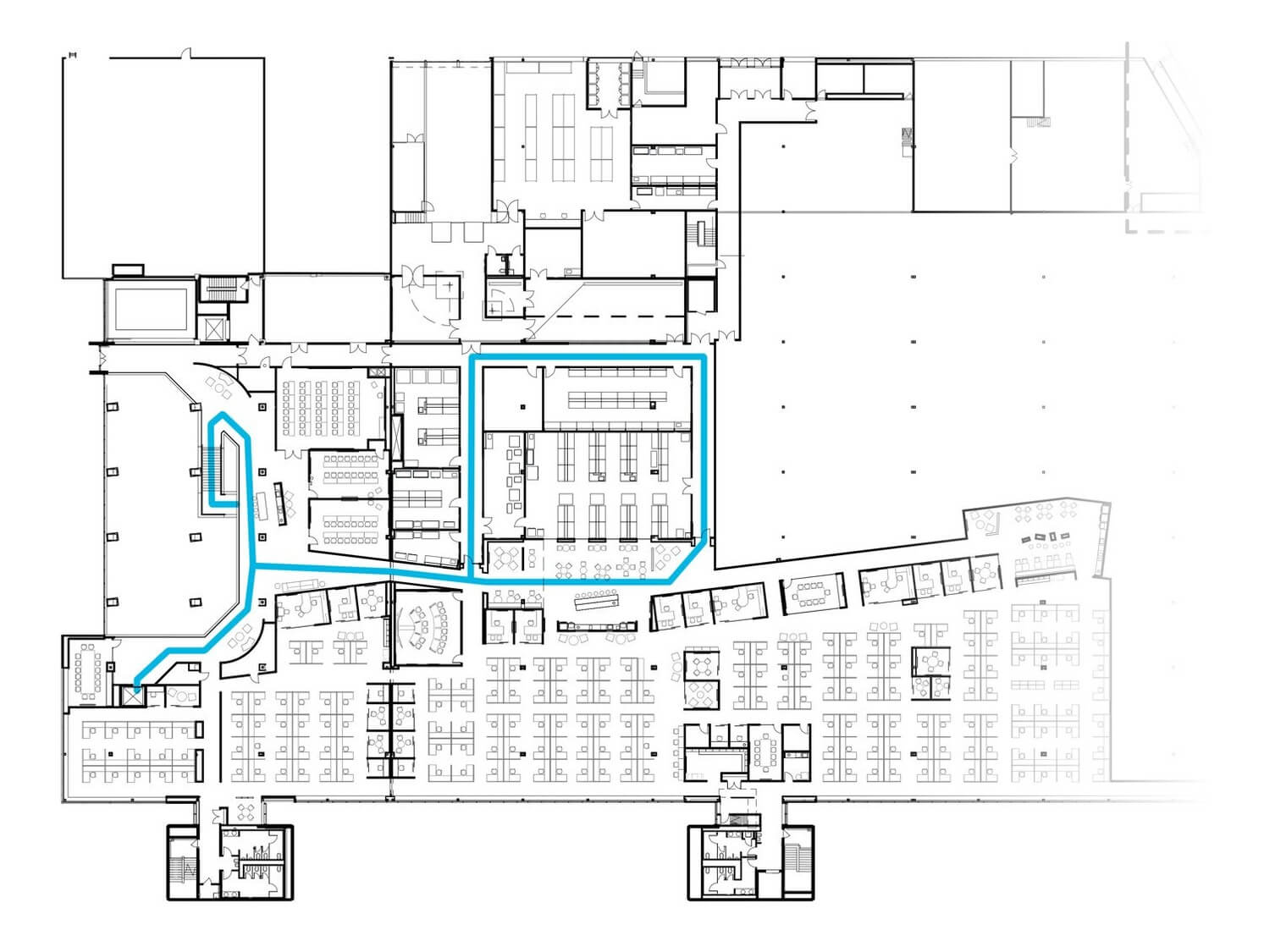
Visitors move through an innovative learning and sales environment.
Customers train on new equipment and partner with Cytiva to develop new drugs. When touring the labs, they follow a discreet route that showcases manufacturing and non-secure laboratory spaces without compromising other customers’ business interests. Educational and marketing materials are located along the tour route in the forms of electronic and traditional media.

Break areas between office and lab spaces encourage interaction among teams.




